Pain Pathway and Classification of Pain
Introduction
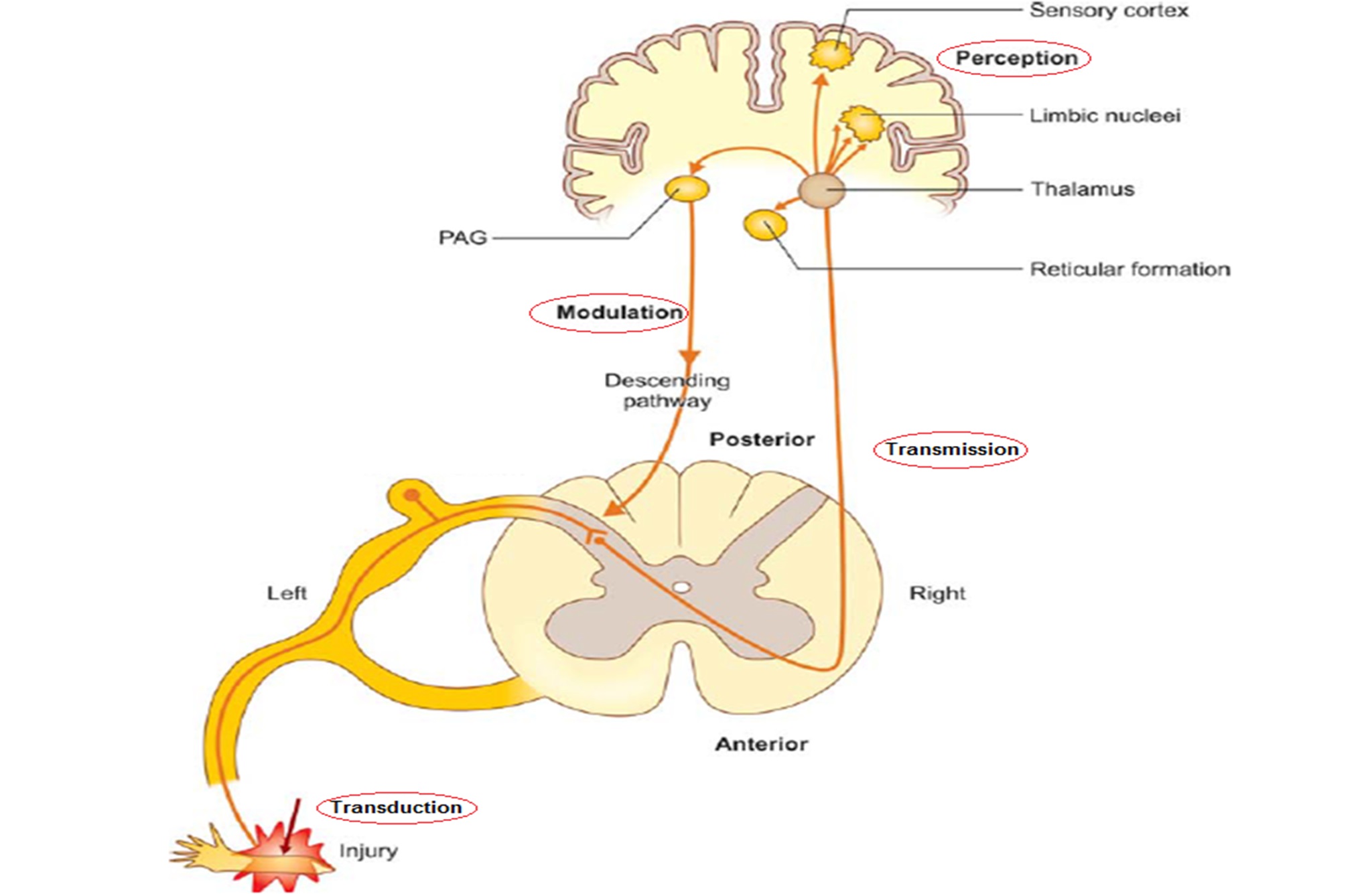
Pain, an intricate sensory experience, involves various physiological and neurological processes. Understanding the pain pathway —comprising transduction, transmission, perception, and modulation—sheds light on its complexities and mechanisms.
Pain Pathway: Understanding Transduction, Transmission, Perception, and Modulation
Transduction:
Pain begins with transduction, where noxious stimuli activate specialized receptors called nociceptors. These receptors convert mechanical, thermal, or chemical stimuli into electrical signals, initiating pain.
Transmission:
Nociceptive signals travel along nerve fibers—Aδ and C fibers—towards the spinal cord and ascend to the brain. Ascending pathways include the spinothalamic tract and the trigeminothalamic tract, transmitting pain signals to higher brain centers for processing.
Perception and Brain Centers:
- Postcentral Gyrus: This area in the parietal lobe receives and processes somatosensory information, including pain perception.
- Limbic System: Emotional and affective responses to pain occur here, contributing to the subjective experience of pain.
- Reticular Formation: Integrates sensory information, modulating arousal and attention related to pain stimuli.
- Periaqueductal Grey Matter (PAG): An integral part of the descending pain modulation system, involved in the regulation of pain perception.
Pain Modulation:
Pain modulation mechanisms aim to alter pain signals, regulating their intensity and perception:
- Descending Inhibitory Pathway: Descending pathways from the brainstem, including the dorsolateral funiculus, modulate pain signals. These pathways release inhibitory neurotransmitters like serotonin and norepinephrine, reducing pain transmission.
- Gate Control Theory: Proposed by Melzack and Wall, this theory suggests a spinal gate mechanism where non-painful input (e.g., rubbing an injured area) can inhibit the transmission of pain signals, altering perception. Spinal Cord Stimulation is developed based on this theory.
- Endogenous Opioids: The body’s natural pain-relieving chemicals, including endorphins and enkephalins, bind to opioid receptors, reducing pain signals and influencing pain perception.
- Cannabinoids: The endocannabinoid system plays a role in pain modulation by regulating neurotransmitter release, exerting anti-inflammatory effects, and modulating pain perception in various pain states.
- Segmental Inhibition: Local inhibitory interneurons in the spinal cord, activated by non-noxious stimuli, inhibit the transmission of pain signals at the segmental level, modulating pain perception.
Summary of pain pathway:
Understanding the pain pathway from transduction to modulation provides insights into the intricacies of pain processing. The involvement of various brain centers, descending pathways, endogenous mechanisms, and modulation theories highlights the complexity of pain perception and offers potential targets for therapeutic interventions.
This chapter aims to elucidate the multifaceted nature of the pain pathway, emphasizing the interplay between sensory input, neurological processing, and modulation mechanisms in shaping the experience of pain.
Classification of Pain: Understanding Mechanisms and Timeframes
Pain, a complex sensory experience, can be classified based on both its underlying mechanisms and its temporal nature. Understanding these classifications provides insight into the diverse nature of pain and aids in tailored management strategies.
Mechanism-Based Classification:
- Nociceptive Pain:
- Mechanism: Arises from the activation of nociceptors in response to tissue damage or inflammation.
- Examples: Pain from cuts, burns, fractures; protective in nature.
- Neuropathic Pain:
- Mechanism: Results from damage or dysfunction in the nervous system, causing aberrant signaling.
- Examples: Post-herpetic neuralgia, phantom limb pain, diabetic neuropathy; often described as shooting, burning, or electric shock-like pain.
- Nociplastic Pain:
- Mechanism: Represents altered pain processing in the absence of ongoing tissue damage or inflammation.
- Examples: Fibromyalgia, complex regional pain syndrome (CRPS); characterized by widespread pain and sensitivity.
- Mixed Pain:
- Mechanism: Occurs when multiple pain mechanisms coexist, such as nociceptive and neuropathic components.
- Examples: Conditions like osteoarthritis with nerve involvement; a combination of tissue damage and nerve dysfunction.
Time-Based Classification:
- Acute Pain:
- Duration: Typically short-lived, lasting up to three to six months.
- Cause: Often associated with tissue damage, surgery, or injury; serves as a protective mechanism.
- Chronic Pain:
- Duration: Persists beyond the expected healing time (more than three to six months).
- Cause: May result from underlying conditions, nerve damage, or alterations in pain processing.
Sensitization and its Impact:
Sensitization refers to an increased responsiveness of neurons to stimuli, amplifying pain signaling. It can occur at both peripheral and central levels:
- Peripheral Sensitization:
- Components: Involves changes in nociceptors at the injury site, leading to increased sensitivity to stimuli (hyperalgesia) and pain from normally non-painful stimuli (allodynia).
- Result: Enhanced input to the spinal cord, contributing to amplified pain signaling.
- Central Sensitization:
- Components: Involves changes in the spinal cord and brain, leading to amplified transmission and processing of pain signals.
- Result: Heightened pain perception, reduced pain threshold, and spread of pain beyond the initial site.
Results of Sensitization:
- Increased Pain Sensitivity: Heightened response to painful stimuli.
- Pain Spread: Expansion of pain to areas beyond the initial site (secondary hyperalgesia).
- Altered Pain Perception: Changes in how the brain processes and perceives pain, leading to persistent discomfort.
Conclusion:
Classifying pain based on mechanisms (nociceptive, neuropathic, nociplastic, mixed) and timeframes (acute, chronic) provides a framework for understanding the diverse nature of pain experiences. Sensitization, both peripheral and central, plays a pivotal role in amplifying pain signals, contributing to increased pain sensitivity and altered pain perception, thereby shaping the chronicity and severity of various pain conditions. Understanding these classifications and the impact of sensitization aids in tailoring effective pain management strategies.


